By Lewis Perdue and Rebecca L. Yeamans
NOTE: An earlier version of this paper originally appeared on nano-active.com on Feb., 24, 2014.
This version is enormously expanded to address peer-review suggestions from multiple prominent researchers. Those who helped improve this paper who wish to remain un-named because the direct and un-nuanced data and opinions expressed here may cause funding, political or other undesirable issues.
However, we are enormously grateful for their help, assistance and encouragement.
Please click this link for a .pdf version of this post.
Bisphenol A (BPA) has become nearly ubiquitous in our environment and can be found in many different products, including the plastic in water bottles and baby bottles, thermal paper for printers, and even in dental sealants and medical devices including intravenous fluid and chemotherapy bags and tubing(26,27,28,29,30,31).
Thousands of studies have been published that examine the levels of BPA and other endocrine disruptors present in the environment along with the mechanisms and pathways those chemicals may take to affect or harm living things.There is, however, considerable controversy concerning negative health effects of so-called “low-dose” exposures.
One recent study, published in the journal Toxicological Sciences, stated as its goals the desire to help shed light on the conflicting body of evidence. Unfortunately, that study, “Toxicity Evaluation of Bisphenol A Administered by Gavage to SPRAGUE-DAWLEY Rats from Gestation Day 6 through Postnatal Day 90(1)“is fatally flawed.
The study(1) by Delclos et al., concluded that low-dose BPA is not harmful. However, that conclusion cannot be scientifically justified by the experiment as conducted. This study suffers from numerous and massive errors including; by the eventual admission of the authors(1); the violation of one of the scientific method’s paramount experimental rules: the control subjects were contaminated by the chemical substance being evaluated.
Additionally, while the study authors claim to have no idea of the BPA contamination source(1), an examination of the paper and a current understanding of the science indicate numerous avenues of contamination that are obvious, well-established, and should have been considered in any careful experiment design and conduct.
This letter will explore a number of those sources and their consequences in more detail below, but it merits consideration at this stage that one possible contamination source could jeopardize results of experiments underway in the very substantial, multi-agency program known as the Consortium Linking Academic and Regulatory Insights on BPA Toxicity (CLARITY-BPA).
Test Animal Contamination Concern For CLARITY-BPA
Delclos et al. state that the test animals in their experiment were obtained from the NTP breeding colony(1). If so, then their contaminated controls may also hold significance for the scores of investigators participating CLARITY-BPA.
“CLARITY-BPA is a collaborative effort between NTP and NIEHS with support from FDA to conduct a perinatal 2-year guide- line chronic rodent toxicity study on BPA(95).”
The program is designed to investigate “scientific uncertainties about BPA’s health effects to better inform regulatory decision-making. …The CLARITY-BPA consortium is made up of NCTR staff, NIEHS and NTP staff, and 12 extramural grantees(95).”
The core guideline- compliant study is being conducted at FDA’s National Center for Toxicological Research (NCTR) CLARITY-BPA investigators, both with government at universities, must use rats from the NTP breeding colony(95).”
The unknown source of the BPA contamination of controls raises the issue of whether the animals in the NTP breeding colony are regularly monitored for BPA and other endocrine disruptors.
Ongoing and regular population sampling by NTP would alert breeders to potential contamination. Prior to release, batch samplings would assure the receiving investigators of the purity of their test animals.
It is unknown whether the NTP breeding colony has monitoring protocols in place that adequately monitor test animals and assure investigators that the test subjects are uncontaminated and appropriate for experimentation.
Avoidable Errors Accumulate
In addition to the contamination error, the study fails on an accumulation of other serious errors:
- Potential contamination by other endocrine-disrupting compounds
- Unknown Combination Effects Of Stressors
- Use of oral gavage which introduces non-chemical stressors
- Use of oral gavage which fails to recreate appropriate BPA Absorption
- Inappropriate Rat Strain
- Poor choice of outcome measures
- Failure to use current science in BPA and Endocrine Disruptor Compound (EDC) research
- Erroneous claim that BPA is “weak” estrogen
- Failure to identify well-known exogenous source(s) of BPA contamination
- Failure to identify well-known exogenous source(s) and estrogenic activity of equipment and supplies
- Failure to measure BPA levels in any of the animals except in the test cohort at the end of the trial
- Failure to measure other exogenous estrogenic compounds in test animals
- Use of oral gavage
- Wrongly examining toxicity when timing is a bigger issue
- Failure to include disclosure of conflicts
Contaminated controls
A negative or naïve control is defined as a group of experimental units that have not had any experimental treatment applied, nor possess any quality that would have an effect on the results of the study. In the study under the microscope(1), in order to be a true negative control, all animals would need to be devoid of any BPA or other endocrine disruptors in their body that interact with or act similar to BPA. Unfortunately, this was not done, thereby rendering all further experimental results invalid(117). The paper itself stated that:
“BPA-glucuronide was detected in the serum of vehicle and naïve control animals at levels similar to those detected in animals dosed with 2.5 ug BPA/kg bw/day. The presence of BPA-glucuronide, which is formed by metabolism of aglycone BPA in the body, in the serum of control animals implies unintentional exposure of the animals to BPA rather than post-sampling contamination of the serum samples. The source of this exposure has not been definitively identified. Thus, the 2.5 μg BPA/bw/day dose group is not distinguishable from the two negative control groups.”(1)
This finding automatically violates one of the most important functions of a control, in that no longer can the researchers determine if the low-dose BPA has an effect on the specific endpoints described in the paper(2,38), since all rats, even the supposed negative controls were contaminated with BPA prior to experimentation.
If there are no differences in the low-dose BPA group and the control group, it is impossible to make the conclusion that low-doses of BPA do not have any effect on the rats when there is no real naïve control to compare to in the first place(2,38,39,40). Additionally, without the comparison of a positive control (i.e. a control treated with BPA, in this case, that is known to promote adverse effects), it is even more difficult to determine if the negative result is real, or if it is a false negative(3,35,39).
Unknown Combination Effects of Stressors
For the purpose of this and other tests of endocrine disruptors, it would not only be necessary to assure that the controls were not contaminated by the substance being investigated, but also for the presence of other endocrine disruptors as well.
Numerous recent studies have raised the confounding problems resulting from unknown antagonistic, agonistic, synergistic interactions which may possibly mask effects from a single compound under study(96,97,98,99).
The National Institute of Environmental Health Sciences (NIEHS) has recognized this issue. In its 2012-2017 Strategic plan, the NIEHS stated that resolving the effects of interactions resulting from combinations of both chemical and non-chemical stressors was a high priority(100).
The NIEHS inclusion of non-chemical stressors speaks as well to gavage-induced stress which results in hormone releases that may alter the effects of a studied compound such as BPA.
Use of oral gavage which introduces non-chemical stressors
In addition to effects relating to internal concentrations of BPA or other compounds active at low doses, gavage can induce the production of stress hormones and alter the internal homeostasis of test animals(101,102,103,104). A very recent BPA-related study that found “potential for gavage to influence gene expression in the developing brain”(105).
Altering the endocrinal homeostasis of test animals through experiment procedures with unknown or equivocal results may initiate undesirable phenomena with the potential to impact an experiment’s overall conclusions. This could be significant in cases where relevant concentrations of hormones and studied compounds are active in nano- or pico- concentrations.
Use of oral gavage which fails to recreate appropriate BPA Absorption
The Delclos et al. study used oral gavage dosing of pregnant rats during the experiment(1). Recent studies in BPA research have found that the oral gavage method may be an inappropriate technique for BPA and other EDC experiments, as this method does not accurately represent the actual ingestion and absorption process by rodents and humans(16,17,18,83,85).
Specifically, “most previous studies have relied on the gavage method, where BPA is distributed evenly in the gut. This is not how food actually enters our bodies. We chew it, move it around in our mouths, and it interacts with numerous surfaces–our tongue, cheeks, etc–before it enters the stomach.”(16). In other words, using the gavage method would suggest that there shouldn’t be any unmetabolized BPA in the blood after exposure, however many studies find that, in fact, there is(16,17,83,84).
In a study using a dog model, researchers compared gavage methods of BPA delivery versus sublingual exposure methods, and found that BPA absorption into the body was significantly higher using sublingual methods than gavage techniques(17) Specifically, the levels of BPA in the dogs’ bodies were significantly higher in those dogs’ receiving the sublingual BPA treatment than the levels of BPA in those dogs’ receiving BPA through gavage. The study concluded that BPA is more efficiently and rapidly absorbed through the mucosa of the mouth, resulting in much higher BPA levels in the body than using gavage methods(17).
This means that a study using gavage results in BPA levels in the bodies of their test subjects that are significantly lower than they would be in a more “real world” scenario such as sublingual absorption and therefore the conclusions from which would be inaccurate. Similar studies in the mice model have found similar results, in that exposure of BPA through consumption of a diet treated with BPA results in a more natural route of exposure than through oral gavage(18,86).
This ad-free article is made possible by the financial support of the
Center for Research on Environmental Chemicals in Humans: a 501(c)(3) non-profit.
Please consider making a tax-deductible donation for continued biomedical research.
Inappropriate Rat Strain
It has been found that there are clear species and strain-specific differences in sensitivity to various estrogenic compounds and EDCs (4,5,41, 42,44,45), so using an animal model that has been shown to respond to the compound(s) in question is crucial for accurate interpretations of results. Specifically, depending upon the strain of rat utilized, results for similar experiments have been found to be markedly different.
Different strains of rats appear to have varying sensitivities to compounds with various estrogenic activities, for example with Charles River Sprague-Dawley rats, studies have found that they are very insensitive to BPA and other estrogenic compounds, thus using them in low-dose studies is inappropriate and not representative of how the compounds act in more sensitive strains or species(4,5,6,43,44,45) In the current FDA study under review, they chose to use Sprague-Dawley rats as their animal model(1).
According to the results of one meta-analysis of all BPA literature through the end of 2004, 90% of government-funded peer-reviewed research articles found adverse effects of BPA exposure(5). A side note: the same meta-analysis discovered that of the industry-funded research articles on BPA, 0% found any adverse effects of BPA exposure, which raises more red flags(5).
Taking only the government-funded peer-reviewed research articles in consideration, when those studies that used Charles River Sprague-Dawley rats were removed from the meta-analysis, the number of studies finding adverse effects of BPA exposure increased from 90% to 96%(5). This jump indicates that the use of the appropriate species be used in BPA experiments, as some strains (including Charles River Sprague-Dawley rats) are not sensitive enough to accurately reflect what could be occurring in the human model(6).
Poor choice of outcome measures
Another flaw with the FDA study is that they chose to focus primarily on the toxic effects of BPA exposure on a set of anatomical outcome measures that has been shown to be unacceptable and unrepresentative of BPA or other EDC exposure over the years(6,7). A major part of the problem is that studies by federal agencies are required to follow OECD Guidelines (OECDG), which often necessitates the use of multi-generational tests in order to stand up in a court of law if needed(6).
The problem with OECDG multi-generational testing regimes is that they use out-of-date testing methods and outcome measures which do not fully capture the effects of BPA exposure, and completely ignore the more contemporary methods and outcome measures like gene expression, DNA methylation, cellular pathways, or epigenetics(6,7).
Specifically, the FDA study ignored current science that indicates that DNA methylation and other epigenetic effects along with interference with cellular signaling pathways are responsible for more relevant biological phenomena that affect obesity(47,48,49) , cancer(50,51,52), Alzheimer’s Disease(53,54,55) and other maladies promoted by BPA and other EDCs(56,57,58,59,60).
Including the FDA study current under review, these types of studies focus mainly on anatomical measurements, gross pathological observations, and other macroscopic outcome measures(1). Since the 1990s, there have been vast improvements in BPA and EDC research methods, with the understanding that out-of-date OECDG methods established as early as the 1950s are not sensitive enough to detect effects of low-dose exposure of BPA and other EDCs. However, federal regulatory agencies still insist on following out-of-date protocols that were created prior to the improvements in our understanding of BPA and EDC exposure on more sensitive measures (DNA methylation, cellular signaling pathways, etc.)(7,61,62).
Failure to use current science in BPA and EDC research
Even though OECDG-based testing regimes do not accurate reflect the harmful public health hazards of BPA or other EDC exposures, federal regulatory outcomes and decisions are still based on those studies using OECDG methods and not based on the many hundreds of academic studies that use more modern techniques and have overwhelmingly found harmful effects of BPA and other EDCs on various human health aspects(6,7,26,46,61,62,63,64).
Both the United States FDA and European Food Safety Authority have in recent years published information showing that their conclusions regarding the safety of BPA has been based solely on the results of a small number of studies following OECD guidelines, while ignoring the expert advice and research of over 700 peer-reviewed academic articles as of 2006(6,7). Since that time, there have been many hundreds more articles demonstrating harmful public health effects of BPA and other EDCs, while the FDA and other federal regulatory agencies continue to ignore this mountainous evidence and respond with their own poorly-designed experiments and out-of-touch science(6,7,26,46,61,62,63,64).
Erroneous claim that BPA is “weak” estrogen
It was originally thought that BPA was a relatively weak estrogenic compound, evidence is mounting that BPA is just as strong an estrogenic compound than natural hormones like estradiol(5,32,36). In other words, what was thought of as being a relatively benign, BPA can actually cause significant problems at the levels currently present in the environment including but not limited to cancer, low testosterone and low sperm motility, miscarriages, and birth defects(23,33,34,35,37).
In the investigation by Delclos et al., the authors stated that “BPA-induced effects partially overlapped those induced by EE2 estradiol, consistent with the known weak estrogenic activity of BPA” (1). While this may have been the understanding very early on in BPA-related research(65,66,67), a significant amount of research has been done since then that refutes this “weak” claim. In fact, many studies are now finding that BPA can elicit estrogen-like activity that has been shown to be as strong as, if not stronger, than EE2, the mechanisms by which are different than “traditional” EE2 pathways(8,9,68,69,70).
Specifically, more recent studies have found that BPA promotes very fast responses, even at low doses, via “non-classical estrogen pathways” and the activation of many different transduction signaling pathways in different cell types which when combined results in greater effects related to gene expression triggered by estrogenic action(8,9,10,71,72).
Failure to identify well-known exogenous source(s) of BPA contamination
Feed: One potential source of BPA contamination in the rats in this and many other EDC research studies is the specific diet or feed that is provided to the animals. The FDA paper itself acknowledged this piece of information and stated that “since many of the effects of BPA are thought to occur through interference with estrogen signaling pathways, a soy- and alfalfa-free diet was used and dietary levels of the phytoestrogens genistein and daidzein were monitored, in addition to BPA.”
In fact, most of commercial rodent feed contains the phytoestrogens genistein and daidzein, which show a strong affinity for estrogen receptor beta and have been shown to be, at times, more potent than the effects of environmental estrogens such as DDT and BPA(11,12,73,74). Studies have confirmed that it is the feed itself that can activate estrogen receptors as a result of estrogenic compounds in the feed, and that it is not due to the metabolism of the food in the gut(21,75,76).
While they were careful to choose an appropriate diet for minimizing phytoestrogen content in the rodent diets during the experiment, they failed to take into consideration the diet the rats received prior to starting the experiment. In the FDA paper, it was stated that “while in the breeding colony, [the breeders] were maintained with their dams on NIH-41 irradiated feed pellets” from Harlan Laboratories(1).
Coming directly from the Harlan Laboratories website, it does not appear that the NIH-41 diet is phytoestrogen-free. Specifically “the diets 2014, 2016, 2019, and 2020X while not completely “free” are best described as minimal phytoestrogen diets. Quarterly testing of these diets for isoflavones (phytoestrogens from soybean) typically reveals levels ranging from less than detectable to 20 ppm. For some perspective, most traditional rodent diets contain from 200 to 500 ppm isoflavones. Phytoestrogen minimal diets such as 2014, 2016, 2019, and 2020X are generally acceptable for studies where phytoestrogens are of concern.”(13)
In other words, NIH-41 from Harlan Laboratories does not appear to be one of their phytoestrogen minimal diets, indicating that prior to experimentation, the rats at the facility used by the FDA study were, in fact, exposed to a range of phytoestrogens on a daily basis, thus indicating a possible source for the pre-experimental contamination of the rats. After getting in touch directly with Harlan Laboratories about NIH-41, they confirmed that this particular feed contains soy-based products (5% soybean meal as well as 2% soy oil), thus confirming the potential for exogenous phytoestrogen exposure prior to testing(25,77).
In regards to the experimental food, while it was reported to be free of the phytoestrogens genistein and daidzein, it was also reported by the FDA researchers to contain “BPA levels below the average analytical blanks.”(1) Even though they controlled for certain phytoestrogens in the animal feed, BPA itself was found in the diet and thus a possible contributor to the BPA contamination of the rats during experimentation(3,25,77).
Finally, since recent research has indicated that the half-life of BPA may be longer than initially thought(6,14,78), the low levels of BPA in the feed could be more of a problem than the FDA researchers assumed.
Failure to identify well-known exogenous source(s) of BPA and estrogenic activity of equipment and supplies
Use of polysulfone cages: In addition to the feed, there are many other exogenous sources of estrogens that the rats were exposed to both prior to and during experimentation by the FDA researchers.
Specifically, laboratory animals are in other words inundated with possible sources of BPA and other EDC exposure, including from bedding, cages, water bottles, water, and disinfectant cleaners(6,11,79,80,81). According to the FDA researchers, their rats were housed in polysulfone cages. While they indicated that equipment used was “low BPA” and that levels were monitored regularly, it wasn’t clear what those values actually were.
Polysulfone pellets are commonly used in many types of equipment for both research and medicine. In fact, one study focusing on kidney hemodialyzers found that polysulfone pellets, which are major components of the hemodialyzer machines, leach a significant amount of BPA into the environment(15,82).
When combined to create the final machine, the polysulfone does not appear to leach as much BPA as it did when in the pellet form; however, it is possible that after long term exposure, patients requiring prolonged use of this polysulfone-based equipment could be adversely affected(15,82).
Finally, there was a failure on the part of the FDA researchers to test the estrogenic activity of all these aforementioned possible exogenous sources of estrogens. It is clear they were not in touch with the current state of EDC research, as they would have been more careful testing the estrogen content of everything the rats came in contact with prior to and during experimentation, since many studies have found vast variability in the sources of possible EDC contamination that must be minimalized as much as possible in BPA and other EDC experiments.
Failure to measure BPA levels in any of the animals except in the test cohort at the very end of the trial
By not measuring BPA levels of any of the animals from the very beginning, and only measuring BPA levels at the very end of the trial, effectively no consideration was given to actual levels of BPA in the animals. Without having a starting point to compare to, it’s near impossible to draw any conclusions based on what was seen at the end(38). If the researchers had tested the animals at the very beginning, they would have found that all animals were contaminated with BPA, even the controls, and at that point some amendments to the protocol could be made.
In addition, failure to measure BPA levels in test animals makes it impossible to measure (as opposed to estimate ) serum concentrations and their possible correlation to effects or to whether the method used for administering the BPA (oral gavage) may have been flawed.
Wrongly examining toxicity when timing is a bigger issue
One problem that had plagued early BPA and EDC research was examining these compounds in terms of their toxicity, while advancements in this type of research has demonstrated that the timing of the exposure is markedly more critical than simple toxicity alone. Cytotoxicity functions at a coarser level in which cell death and malfunction can be easily observed. Endocrine disruptors function at the level of hormone receptors(105, 107-110) and also exhibit measurable epigenetic effects(111-113), and affect gene expression(114,115).
In other words, a certain level of BPA exposure to an adult may not have much of an effect, while if that same level of BPA is exposed to a developing embryo at a very specific point in its development, a cascading series of events can be “programmed” into the embryo that could develop into significant health problems later in life(4,22,23,24,87,88).
There is also evidence in the literature that the adult endocrine system is able to maintain a sort of homeostasis, or regulation of a constant balanced environment, thereby may not be as sensitive to the effects of EDC exposure as say if it were going through puberty, a state of marked hormonal flux, or embryonic development, both which have been shown to be significantly more sensitive to BPA and other EDC exposure at those specific times(4,24,89,90,91,92).
Choosing an outdated model such as “toxicity” is inappropriate when timing is what is most crucial in determining adverse effects of BPA and other EDC exposure.
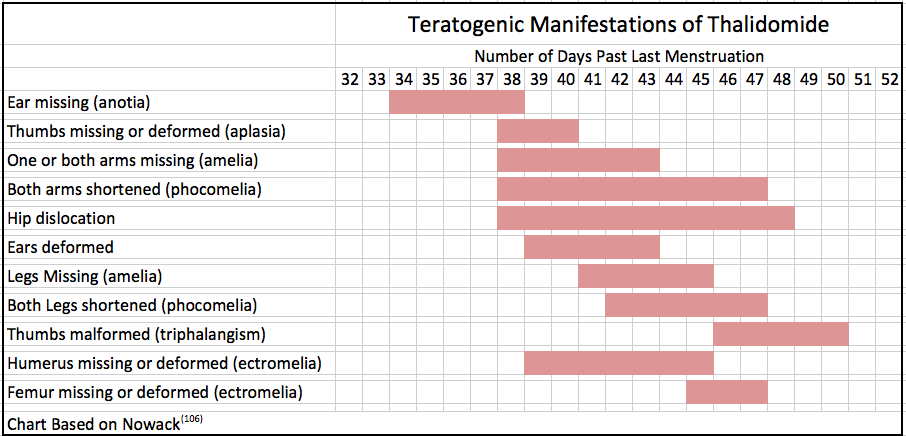
Significantly, the drug Thalidomide passed traditional toxicity testing and yet caused one of the most prominent pharmaceutical disasters in history(116).
One important lesson from the Thalidomide disaster concerns the timing of exposures since BOA and other EDCs can affect hormones, especially in developing As can be seen in the following chart, the drug’s effects resulted in very different deleterious effects depending upon the time of exposure.
The lesson to be learned is that the evaluation of endocrine disrupting compounds needs to go beyond traditional toxicity testing. It needs to be tested at a wide variety of relevant concentrations and be thoroughly evaluated at many different timing intervals of exposure using the most sensitive tests possible
Failure to disclose conflicts of interest
Conflicts of interest, while seemingly short and often times an after-thought for some researchers, is a critical part of the publication process. Even if there are no conflicts of interest, it is in the researcher’s best interest to provide this information. The existence of conflicts of interest may negatively influence the results of the study in question, essentially invalidating the conclusions presented(19,93,94).
By disclosing any conflicts, or indicating “none” if that happens to be the case, important information is presented to the reader so that they may be able to cast their own judgment on the legitimacy and objectiveness of the researcher’s results and conclusions(20,93,94) . A failure to disclose conflicts of interest casts a skeptical eye on the validity of the entire study even when the experiment is conducted properly.
Conclusions
Given the number of failures, it is hard to see how this paper can be corrected, especially with a tainted data set. The only alternative is for it to be re-done with the failings corrected and a new, valid set of data.
The only other conclusion that can be drawn about a study with so many crippling, obvious, and avoidable flaws is that the regulatory agencies of the U.S. government need to raise their standards to match those of the National Institutes of Health.
NIH has three stringent levels of scrutiny involving peer review: Before grants are awarded, the competency of the investigators is assessed as are, experiment design, appropriate outcomes and use of the most current science and lab techniques(7). This is followed by post-investigation peer-review, publication in peer-reviewed journals and efforts to follow up the study with replication and updated results. Conflicts of interest are required to be declared.
Declaration of Conflicts of Interest
Neither Lewis Perdue nor Rebecca L. Yeamans have any conflicts of interest to disclose. They claim to be able to speak freely without risking project funding because they have no grants or compensation of any form from any source, either private or public.
==========================================================
REFERENCES
(1) Delclos, K.B., Camacho, L., Lewis, S.M., Vanlandingham, M.M., Latendresse, J.R., Olson, G.R., Davis, K.J., Patton, R.E., da Costa, G.G., Woodling, K.A., Bryant, M.S., Chidambaram, M., Trbojevich, R., Juliar, B.E., Felton, R.P., and Thorn, B.T. 2014. Toxicity evaluation of Bisphenol A administered by gavage to Sprague-Dawley rats from gestation day 6 through postnatal day 90. Toxicological Sciences doi: 10.1093/toxsci/kfu022
(2) Johnson, P.D., and Besselsen, D.G. 2002. Practical aspects of experimental design in animal research. ILAR Journal (Institute for Laboratory Animal Research) 43(4): 202-206.
(3) Vom Saal, F.S., Richter, C.A., Ruhlen, R.R., Nagel, S.C., Timms, B.G., and Welshons, W.V. 2005. The importance of appropriate controls, animal feed, and animal models in interpreting results from low-dose studies of Bisphenol A. Birth Defects Research 73: 140-145.
(4) Vandenberg, L.N., Colborn, T., Hayes, T.B., Heindel, J.J., Jacobs, Jr., D.R., Lee, D-H., Shioda, T., Soto, A.M., vom Saal, F.S., Welshons, W.V., Zoeller, R.T., and Myers, J.P. 2012. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocrine Reviews 33: 0000-0000.
(5) vom Saal, F.S., and Hughes, C. 2005. An extensive new literature concerning low-dose effects of Bisphenol A shows the need for a new risk assessment. Environmental Health Perspectives 113(8): 926-933.
(6) Hunt, P.A., Susiarjo, M., Rubio, C., and Hassold, T.J. 2009. The BPA Experience: A primer for the analysis of environmental effects on mammalian reproduction. Biology of Reproduction 81: 807-813.
(7) Myers, J.P., vom Saal, F.S., Akingbemi, B.T., Arizono, K., Belcher, S., Colborn, T., Chahoud, I., Crain, D.A., Farabollini, F., Guillette, Jr., L.J., Hassold, T., Ho, S., Hunt, P.A., Iguchi, T., Jobling, S., Kanno, J., Laufer, H., Marcus, M., McLachlan, J.A., Nadal, A., Oehlmann, J., Olea, N., Palanza, P., Parmigiani, S., Rubin, B.S., Schoenfelder, G., Sonnenschein, C., Soto, A.M., Talsness, C.E., Taylor, J.A., Vandenberg, L.N., Vandenbergh, J.G., Vogel, S., Watson, C.S., Welschons, W.V., and Zoeller, R.T. 2009. Why public health agencies cannot depend on Good Laboratory Practices as a criterion for selecting data: The case of Bisphenol A. Environmental Health Perspectives 117(3): 309-315.
(8) Alonso-Magdalena, P., Ropero, A.B., Soriano, S., García-Arévalo, M., Ripoll, C., Fuentes, E., Quesada, I., and Nadal, A. 2012. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Molecular and Cellular Endocrinology 355: 201-207.
(9) Hugo, E., Brandebourg, T.D., Woo, J.G., Loftus, J., Alexander, J.W., and Ben-Jonathan, N. 2008. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environmental Health Perspectives 116 (12): 1642-1647.
(10) Watson, C.S., Bulayeva, N.N., Wozniak, A.L., and Finnerty, C.C. 2005. Signaling from the membrane via membrane estrogen receptor-α: Estrogens, xenoestrogens, and phytoestrogens. Steroids 70: 364-371.
(11) Thigpen, J.E., Setchell, K.D.R., Saunders, H.E., Haseman, J.K., Grant, M.G., and Forsythe, D.B. 2004. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. ILAR Journal 45(4): 401-416.
(12) Shelby, M.D., Newbold, R.R., Tully, D.B., Chae, K., and Davis, V.L. 1996. Assessing environmental chemicals for estrogenicity using a combination of in vitro and in vivo assays. Environmental Health Perspectives 104(12): 1296-1300.
(13) Harlan Laboratories Diets FAQ page: http://www.harlan.com/products_and_services/research_models_and_services/laboratory_animal_diets/diets_faqs.hl#11 (accessed 02/22/2014).
(14) Stahlhut, R.W., Welshons, W.V., and Swan, S.H. 2009. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environmental Health Perspectives 117(5): 784-789.
(15) Haishima, Y., Hayashi, Y., Yagami, T., and Nakamura, A. 2001. Elution of Bisphenol-A from hemodialyzers consisting of polycarbonate and polysulfone resins. Journal of Biomedical Materials Research58(2): 209-215.
(16) Barrett, J.R. 2013. Oral Argument: Sublingual findings challenge key assumptions about BPA exposure. Environmental Health Perspectives 121(8): A257.
(17) Gayrard, V., Lacroix, M.Z., Collet, S.H., Viguié, C., Bousquet-Melou, A., Toutain, P-L., and Picard-Hagen, N. 2013. High bioavailability of Bisphenol A from sublingual exposure. Environmental Health Perspectives 121(8): 951-956.
(18) Sieli, P.T., Jašarević, E., Warzak, D.A., Mao, J., Ellersieck, M.R., Liao, C., Kannan, K., Collet, S.H., Toutain, P-L., vom Saal, F.S., and Rosenfeld, C.S. 2011. Comparison of serum Bisphenol A concentrations in mice exposed to Bisphenol A through the diet versus oral bolus exposure. Environmental Health Perspectives 119(9): 1260-1265.
(19) Van McCrary, S., Anderson, C.B., Jakovljevic, J., Khan, T., McCullough, L.B., Wray, N.P., and Brody, B.A. 2000. A national survey of policies on disclosure of conflicts of interest in biomedical research. The New England Journal of Medicine 343(22): 1621-1626.
(20) Sass, J. 2009. Effective and practical disclosure policies: NRDC paper on workshop to identify key elements of disclosure policies for health science journals. National Resources Defense Council Report. https://www.nrdc.org/health/disclosure/files/disclosure.pdf?origin=publication_detail Accessed 02/23/2014.
(21) Ciana, P., Brena, A., Sparaciari, P., Bonetti, E., Di Lorenzo, D., and Maggi, A. 2005. Estrogenic activities in rodent estrogen-free diets. Endocrinology 146(12): 5144-5150.
(22) Markey, C.M., Coombs, M.A., Sonnenschein, C., and Soto, A.M. 2003. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evolution & Development 5(1): 67-75.
(23) Markey, C.M., Wadia, P.R., Rubin, B.S., Sonnenschein, C., and Soto, A.M. 2005. Long-term effects of fetal exposure to low doses of the xenoestrogen Bisphenol-A in the female mouse genital tract. Biology of Reproduction 72: 1344-1351.
(24) Marty, M.S., Carney, E.W., and Rowlands, J.C. 2011. Endocrine disruption: Historical perspectives and its impact on the future of toxicological testing. Toxicological Sciences 120(S1): S93-S108.
(25) NIH Specification: NIH 41 Open Formula Rodent Irradiated Diet specs. 2000. http://www.ors.od.nih.gov/sr/dvr/drs/nutrition/Documents/SpecsDiets/41.pdf Accessed 02/24/2014.
(26) Vandenberg, L.N., Hauser, R., Marcus, M., Olea, N., Welshons, W.V. 2007. Human exposure to bisphenol A (BPA). Reproductive Toxicology 24: 139-177.
(27) Nam, S.H., Seo, Y.M., Kim, M.G. 2010. Bisphenol A migration from polycarbonate baby bottle with repeated use. Chemosphere 79: 949-952.
(28) Biedermann, S., Tschudin, P., and Grob, K. 2010. Transfer of bisphenol A from thermal printer paper to the skin. Analytical and Bioanalytical Chemistry 398: 571-576.
(29) Ehrlich, S., Calafat, A.M., Humblet, O., Smith, T., and Hauser, R. 2014. Handling of thermal receipts as a source of exposure of Bisphenol A. JAMA 311(8): 859-860.
(30) Kloukos, D., Pandis, N., and Eliades, T. 2013. In vivo bisphenol-A release from dental pit and fissure sealants: A systematic review. Journal of Dentistry 41: 659-667.
(31) Duty, S.M., Mendonca, K., Hauser, R., Calafat, A.M., Ye, X., Meeker, J.D., Ackerman, R., Cullinane, J., Faller, J., and Ringer, S. 2013. Potential sources of bisphenol A in the neonatal intensive care unit. Pediatrics 131(3): 483-489.
(32) Sharpe, R.M. 2010. Is it time to end concerns over the estrogenic effects of Bisphenol A? Toxilogical Science 114(1):1-4.
(33) Benachour, N., and Aris, A. 2009. Toxic effects of low doses of Bisphenol-A on human placental cells. Toxicology and Applied Pharmacology 241: 322-328.
(34) LaPensee, E.W., Tuttle, T.R., Fox, S.R., and Ben-Jonathan, N. 2009. Bisphenol A at low nanomolar doses confers chemoresistance in estrogen receptor-α-positive and –negative breast cancer cells. Environmental Health Perspectives 117(2): 175-180.
(35) vom Saal, F.S., and Welshons, W.V. 2006. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environmental Research 100: 50-76.
(36) Seidlová-Wuttke, D., Jarry, H., Christoffel, J., Rimoldi, G., and Wuttke, W. 2005. Effect of bisphenol-A (BPA), dibutylphtalate (DBP), benzophenone-2 (BP2), procymidone (Proc), and linurone (Lin) on fat tissue, a variety of hormones and metabolic parameters: A 3 month comparison with effects of estradiol (E2) in ovariectomized (ovx) rats. Toxicology 213: 13-24.
(37) Baldi, E., and Muratori, M. 2013. Genetic damage in human spermatozoa. Advances in Experimental Medicine and Biology 791. 195p.
(38) Cox, D.R., and T., Reid, N. 2000. The theory of the design of experiments. Monographs on Statistics and Applied Probability, 86. Boca Raton: Chapman & Hall/CRC Press.
(39) Hanson, N.2011. Using biological data from field studies with multiple reference sites as a basis for environmental management: The risks for false positives and false negatives. Journal of Environmental Management 92: 610-619.
(40) vom Saal, F.S., Richter, C.A., Mao, J., and Welshons, W. 2005. Commercial animal feed: Variability in estrogenic activity and effects on body weight in mice. Birth Defects Research (Part A) 73: 474-475.
(41) Wiklund, J.A., and Gorski, J. 1982. Genetic differences in estrogen-induced deoxyribonucleic acid synthesis in the rat pituitary: Correlations with pituitary tumor susceptibility. Endocrinology 111(4): 1140-1149.
(42) Wiklund, J.A., Wertz, N., and Gorski, J. 1981. A comparison of estrogenic effects on uterine and pituitary growth and prolactin synthesis in F344 and Holtzman rats. Endocrinology 109(5): 1700:1707.
(43) Diel, P., Schmidt, S., Vollmer, G., Janning, P., Upmeier, A., Michna, H., Bolt, H.M., and Degen, G.H. 2004. Comparative responses of three rat strains (DA/Han, Sprague-Dawley and Wistar) to treatment with environmental estrogens.
(44) Brossia, L.J., Roberts, C.S., Lopez, J.T., Bigsby, R.M., and Dynlacht, J.R. 2009. Interstrain differences in the development of pyometra after estrogen treatment of rats. Journal of the American Association of Laboratory Animals 48(5): 517-520.
(45) Geis, R.B., Diel, P., Degen, G.H., and Vollmer, G. 2005. Effects of genistein on the expression of hepatic genes in two rat strains (Sprague-Dawley and Wistar). Toxicology Letters 157: 21-29.
(46) Richter, C.A., Birnbaum, L.S., Farabollini, F., Newbold, R.R., Rubin, B.S., Talsness, C.E., Vandenbergh, J.G., Walser-Kuntz, D.R., and vom Saal, F.S. 2007. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive Toxicology 24(2): 199-224.
(47) Dick, K.J., Nelson, C.P., Tsaprouni, L., Sandling, J.K., Aïssi, D., Wahl, S., Meduri, E., Morange, P.E., Gagnon, F., Grallert, H., Waldenberger, M., Peters, A., Erdmann, J., Hengstenberg, C., Cambien, F., Goodall, A.H., Ouwehand, W.H., Schunkert, H., Thompson, J.R., Spector, T.D., Gieger, C., Trégouët, D.A., Deloukas, P., and Samani, N.J. 2014. DNA methylation and body-mass index: a genome-wide analysis. The Lancet. DOI: 10.1016/S0140-6736(13)62674-4.
(48) Milagro, F.I., Mansego, M.L., De Miguel, C., and Martínez, J.A. 2013. Dietary factors, epigenetic modifications and obesity outcomes: Progresses and perspectives. Molecular Aspects of Medicine 34: 782-812.
(49) Sales, L.B., Kamstra, J.H., Cenijn, P.H., van Rijt, L.S., Hamers, T., and Legler, J. 2013. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicology In Vitro 27: 1634-1643.
(50) Coppedè, F. 2014. Epigenetic biomarkers of colorectal cancer: Focus on DNA methylation. Cancer Letters 342(2): 238-247.
(51) Kulis, M., Queirós, A.C., Beekman, R., and Martín-Subero, J.I. 2013. Intragenic DNA methylation in transcriptional regulation, normal differentiation and cancer. Biochimica et Biophysica Acta (BBA) – Gene Regulatory Mechanisms 1829(11): 1161-1174.
(52) Towle, R., Truong, D., Hogg, K., Robinson, W.P., Poh, C.F., ad Garnis, C. 2013. Global analysis of DNA methylation changes during progression of oral cancer. Oral Oncology 49: 1033-1042.
(53) Furuya, T.K., Silva, P.N.O., Payāo, S.L.M., Bertolucci, P.H.F., Rasmussen, L.T., De Labio, R.W., Braga, I.L.S., Chen, E.S., Turecki, G., Mechawar, N., Mill, J., and Smith, M.A.C. 2012. Analysis of SNAP25 mRNA expression and promoter DNA methylation in brain areas of Alzheimer’s disease patients. Neuroscience 220: 41-46.
(54) Coppieters, N., Dieriks, B.V., Lill, C., Faull, R.L.M., Curtis, M.A., and Dragunow, M. 2014. Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain. Neurobiology of Aging 35(6): 1334-1344.
(55) Mastroeni, D., Grover, A., Delvaux, E., Whiteside, C., Coleman, P.D., and Rogers, J. 2010. Epigenetic changes in Alzheimer’s disease: Decrements in DNA methylation. Neurobiology of Aging 31(12): 2025-2037.
(56) Skinner, M.K., Manikkam, M., and Guerrero-Bosagna, C. 2011. Epigenetic transgenerational actions of endocrine disruptors. Reproductive Toxicology 31(3): 337-343.
(57) Guerrero-Bosagna, C., Covert, T.R., Haque, M., Settles, M., Nilsson, E.E., Anway, M.D., and Skinner, M.K. 2012. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reproductive Toxicology 34(4): 694-707.
(58) Collotta, M., Bertazzi, P.A., and Bollati, V. 2013. Epigenetics and pesticides. Toxicology 307: 35-41.
(59) Kundakovic, M., and Champagne, F.A. 2011. Epigenetic perspective on the developmental effects of bisphenol A. Brain, Behavior, and Immunity 25(6): 1084-1093.
(60) Vandegehuchte, M.B. and Janssen, C.R. 2014. Epigenetics in an ecotoxicological context. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 764-765: 36-45.
(61) vom Saal, F.S., Akingbemi, B.T., Belcher, S.M., Birnbaum, L.S., Crain, D.A., Eriksen, M., Farabollini, F., Guillette, Jr., L.J., Hauser, R., Heindel, J.J., Ho, S.M., Hunt, P.A., Iguchi, T., Jobling, S., Kanno, J., Keri, R.A., Knudsen, K.E., Laufer, H., LeBlanc, G.A., Marcus, M., McLachlan, J.A., Myers, J.P., Nadal, A., Newbold, R.R., Olea, N., Prins, G.S., Richter, C.A., Rubin, B.S., Sonnenschein, C., Soto, A.M., Talsness, C.E., Vandenbergh, J.G., Vandenberg, L.N., Walser-Kuntz, D.R., Watson, C.S., Welshons, W.V., Wetherill, Y., and Zoeller, R.T. 2007. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reproductive Toxicology 24: 131-138.
(62) Crain, D.A., Eriksen, M., Iguchi, T., Jobling, S., Laufer, H., LeBlanc, G.A., and Guillette, Jr., L.J. 2007. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reproductive Toxicology 24: 225-239.
(63) Keri, R.A., Ho, S.M., Hunt, P.A., Knudsen, K.E., Soto, A.M., and Prins, G.S. 2007. An evaluation of the evidence for the carcinogenic activity of bisphenol A. Reproductive Toxicology 24: 240-252.
(64) Wetherill, Y.B., Akingbemi, B.T., Kanno, J., McLachlan, J.A., Nadal, A., Sonnenschein, C., Watson, C.S., Zoeller, R.T., and Belcher, S.M. 2007. In vitro molecular mechanisms of bisphenol A action. Reproductive Toxicology 24(2): 178-198.
(65) Kuiper, G.G.J.M., Lemmen, J.G., Carlsson, B., Corton, J.C., Safe, S.H., van der Saag, P.T., van der Burg, B., and Gustafsson, J.A. 1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 139(10): 4252-4263.
(66) Andersen, H.R., Andersson, A.M., Arnold, S.F., Autrup, H., Barfoed, M., Beresford, N.A., Bjerregaard, P. Christiansen, L.B., Gissel, B., Hummel, R., Jørgensen, E.B., Korsgaard, B., Le Geuvel, R, Leffers, H., McLachlan, J., Møller, A., Nielsen, J.B., Sonnenschein, N., Soto, A.M., Sumpter, J.P., Thorpe, S.M., and Grandjean, P. 1999. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environmental Health Perspectives 107(1): 89-108.
(67) Fang, H., Tong, W., Perkins, R., Soto, A.M., Prechtl, N.V., and Sheehan, D.M. 2000. Quantitative comparisons of in vitro assays for estrogenic activities. Environmental Health Perspectives 108(8): 723-729.
(68) Alonso-Magdalena, P., Laribi, O., Ropero, A.B., Fuentes, E., Ripoll, C., Soria, B., and Nadal, A. 2005. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic α-cells through a nonclassical membrane estrogen receptor with intact Islets of Langerhans. Environmental Health Perspectives 113(8): 969-977.
(69) Alonso-Magdalena, P., Morimoto, S., Ripoll, C., Fuentes, E., and Nadal, A. 2006. The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environmental Health Perspectives 114(1): 106-112.
(70) Zsarnovszky, A., Le, H.H., Wang, H.S., and Belcher, S.M. 2005. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: Potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen Bisphenol A. Endocrinology 146(12): 5388-5396.
(71) Nadal, A., Ropero, A.B., Laribi, O., Maillet, M., Fuentes, E., and Soria, B. 2000. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. PNAS 97(21): 11603-11608.
(72) Quesada, I., Fuentes, E., Viso-Leon, M.C., Soria, B., Ripoll, C., and Nadal, A. 2002. Low doses of the endocrine disruptor bisphenol-A and the native hormone 17β-estradiol rapidly activate transcription factor CREB. The FASEB Journal 16(12): 1671-1673.
(73) Brown, N.M., and Setchell, K.D. 2001. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Laboratory Investigation 81(5): 735-747.
(74) Thigpen, J.E., Haseman, J.K., Saunders, H.E., Setchell, K.D.R., Grant, M.G., and Forsythe, D.B. 2003. Dietary phytoestrogens accelerate the time of vaginal opening in immature CD-1 mice. Comparative Medicine 53(6): 607-615.
(75) Odum, J., Tinwell, H., Jones, K., Van Miller, J.P., Joiner, R.L. Tobin, G., Kawasaki, H., Deghenghi, R., and Ashby, J. 2001. Effect of rodent diets on the sexual development of the rat. Toxicological Sciences 61: 115-127.
(76) Santell, R.C., Chang, Y.C., Nair, M.G., and Helferich, W.G. 1997. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. Journal of Nutrition 127(2): 263-269.
(77) Boettger-Tong, H., Murthy, L., Chiapetta, C., Kirkland, J.L., Goodwin, B., Adlecreutz, H., Stancel, G.M., and Mäkelä, S. 1998. A case of laboratory animal feed with high estrogenic activity and its impact on in vivo responses to exogenously administered estrogens. Environmental Health Perspectives 106(7): 369-373.
(78) Korn, E.L., and Graubard, B.I. 1991. Epidemiologic studies utilizing surveys: Accounting for sampling design. American Journal of Public Health 81(9): 1166-1173.
(79) Howdeshell, K.L., Peterman, P.H., Judy, B.M., Taylor, J.A., Orazio, C.E., Ruhlen, R.L., vom Saal, F.S., and Welshons, W.V. 2003. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environmental Health Perspectives 111(9): 1180-1187.
(80) Thigpen, J.E., Setchell, K.D.R., Kissling, G.E., Locklear, J., Caviness, G.F., Whiteside, T., Belcher, S.M., Brown, N.M., Collins, B.J., Lih, F.B, Tomer, K.B., Padilla-Banks, E., Camacho, L., Adsit, F.G, and Grant, M. 2013. The estrogenic content of rodent diets, bedding, cages, water bottles, and its effect on Bisphenol A studies. Journal of the American Association for Laboratory Animal Science 52(2): 130-141.
(81) Thigpen, J.E., Joshi, S., Caviness, G.F., Whiteside, T., Locklear, J., Grant, M., and Forsythe, D.B. 2006. Survey of various types of rodent bedding for estrogenic activity. Journal of the American Association for Laboratory Animal Science 45: 87.
(82) Murakami, K., Ohashi, A., Hori, H., Hibiya, M., Shoji, Y., Kunisaki, M., Akita, M., Yagi, A., Sugiyama, K., Shimozato, S., Ito, K., Takahashi, H., Takahashi, K., Yamamoto, K., Kasugai, M., Kawamura, N., Nakai, S., Hasegawa, M., Tomita, M., Nabeshima, K. Hiki, Y., and Sugiyama, S. 2007. Accumulation of Bisphenol A in hemodialysis patients. Blood Purification 25(3): 290-294.
(83) Patel, V.F., Liu, F., and Brown, M.B. 2011. Advances in oral transmucosal drug delivery. Journal of Controlled Release 153: 106-116.
(84) Mielke, H., and Gundert-Remy, U. 2009. Bisphenol A levels in blood depend on age and exposure. Toxicology Letters 190: 32-40.
(85) Vandenberg, L.N., Chahoud, I., Heindel, J.J., Padmanabhan, V., Paumgartten, F.J.R., and Schoenfelder, G. 2010. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to Bisphenol A. Environmental Health Perspectives 118(8): 1055-1070.
(86) Doerge, D.R., Twaddle, N.C., Woodling, K.A., and Fisher, J.W. 2010. Pharmacokinetics of bisphenol A in neonatal and adult rhesus monkeys. Toxicology and Applied Pharmacology 248: 1-11.
(87) Herbst, A.L., and Anderson, D. 1990. Clear cell adenocarcinoma of the vagina and cervix secondary to intrauterine exposure to diethylstilbestrol. Seminars in Surgical Oncology 6(6): 343-346.
(88) Newbold, R.R., Jefferson, W.N., Padilla-Banks, E., and Haseman, J. 2004. Developmental exposure to diethylstilbestrol (DES) alters uterine response to estrogens in prepubescent mice: low versus high dose effects. Reproductive Toxicology 18: 399-406.
(89) Schulz, K.M., Molenda-Figueria, H.A., and Sisk, C.L. 2009. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Hormones and Behavior 55: 597-605.
(90) Schulz K.M., and Sisk, C.L. 2006. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Molecular and Cellular Endocrinology 254-255: 120-126.
(91) Primus, R.J., and Kellogg, C.K. 1990. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Hormones and Behavior 24: 311-323.
(92) Soto, A.M., Rubin, B.S., and Sonnenschein, C. 2009. Interpreting endocrine disruption from an integrative biology perspectives. Molecular and Cellular Endocrinology 304: 3-7.
(93) Hardell, L., Walker, M.J., Walhjalt, B., Friedman, L.S., and Richter, E.D. 2007. Secret ties to industry and conflicting interests in cancer research. American Journal of Industrial Medicine 50: 227-233.
(94) Homer, J., and Minifie, F.D. 2011. Research Ethics III: publication practices and authorship, conflicts of interest, and research misconduct. Journal of Speech, Language, and Hearing Research 54: S346-S362.
(95) Schug, T.T., Heindel, J.J., Camacho, L., Delclos, K.B., Howard, P., Johnson, A.F., Aungst, J., Keefe, D., Newbold, R., Walker, N.J., Zoeller, R.T., and Bucher, J.R. 2013. A new approach to synergize academic and guideline-compliant research: The CLARITY-BPA research program. Reproductive Toxicology 40:35–40
(96) Biemann R., Fischer B., and Navarrete Santos A. 2014. Adipogenic Effects of a Combination of the Endocrine-Disrupting Compounds Bisphenol A, Diethylhexylphthalate, and Tributyltin. Obesity Facts 7:48-56 (DOI:10.1159/000358913)
(97) Webster, T.F. 2013. Mixtures of endocrine disruptors: How similar must mechanisms be for concentration addition to apply? Toxicology 313(2-3): 129–133.
(98) Regnier, S.M., and Sargis, R.M. 2014. Adipocytes under assault: Environmental disruption of adipose physiology. Biochimica et Biophysica Acta 1842: 520–533.
(99) Hu, Y., Wang, R., Xiang, Z., Qian, W., Han, X., and Li, D. 2014. Antagonistic Effects of a Mixture of Low-Dose Nonylphenol and Di-N-Butyl Phthalate (Monobutyl Phthalate) on the Sertoli Cells and Serum Reproductive Hormones in Prepubertal Male Rats In Vitro and In Vivo. PLOS One (DOI: 10.1371/journal.pone.0093425).
(100) Carlin, D.J., Rider, C.V., Woychik, R., and Birnbaum, L.S. 2013. Unraveling the Health Effects of Environmental Mixtures: An NIEHS Priority. Environmental Health Perspectives 121(1): a6–a8.
(101) Brown, A.P., Dinger, N., and Levine, B.S. 2000. Stress Produced by Gavage Administration in the Rat. Journal of the American Association for Laboratory Animal Science 39(1): 17-21.
(102) Walker, M.K., Boberg, J.R., Walsh, M.T., Wolf, V., Trujillo, A., Skelton Duke, M., Palm, R., and Felton, L.A. 2012. A less stressful alternative to oral gavage for pharmacological and toxicological studies in mice. Toxicology and Applied Pharmacology 260(1): 65–69.
(103) Arantes-Rodrigues, R., Henriques, A., Pinto-Leite, R., Faustino-Rocha, A., Pinho-Oliveira, J., Teixeira-Guedes, C., Seixas, F., Gama, A., Colaço, B., Colaço, A., and Oliveira, P.A. 2012. The effects of repeated oral gavage on the health of male CD-1 mice. Lab Animal 41(5):129-134.
(104) Vaughn, E., Sunohara-Neilson, J., Ovari, J., and Leri, F. 2012. Oral Gavage in Rats: Animal Welfare Evaluation. Journal of the American Association for Laboratory Animal Science 51(1): 25–30.
(105) Cao., J., Rebuli, M.E., Rogers, J., Todd, K.L., Leyrer, S.M., Ferguson, S.A., and Patisaul, H.B. 2013 Prenatal Bisphenol A Exposure Alters Sex-Specific Estrogen Receptor Expression in the Neonatal Rat Hypothalamus and Amygdala. Toxicological Sciences 133(1) 157-173.
(106) Nowack, E. 1965. Die sensible phase bei der thalidomid-embryopathie Humangenetik 1(6):516-536.
(107) Chevrier, J., Gunier, R.B., Bradman, A., Holland, N.T., Calafat, A.M., Eskenazi, B., and Harley, K.G. 2012. Maternal Urinary Bisphenol A during Pregnancy and Maternal and Neonatal Thyroid Function in the CHAMACOS Study. Environmental Health Perspectives 121(1): 138–144.
(108) Shen, O., Wu, W., Du, G., Liu, R., Yu, L., Sun, H., Han, X., Jiang, Y., Shi, W., Hu, W., Song, L., Xia, Y., Wang, S., and Wang, X. 2011. Thyroid Disruption by Di-n-Butyl Phthalate (DBP) and Mono-n-Butyl Phthalate (MBP) in Xenopus. PLoS One 6(4): e19159. Published online 2011 April 22. doi: 10.1371/journal.pone.0019159
(109) Schmutzler, C., Gotthardt, I., Hofmann, P.J., Radovic, B., Kovacs, G., Stemmler, L., Nobis, I., Bacinski, A., Mentrup, B., Ambrugger, P., Grüters, A., Malendowicz, L.K., Christoffel, J., Jarry, H., Seidlovà-Wuttke, D., Wuttke, W., and Köhrle, J. 2007. Endocrine Disruptors and the Thyroid Gland—A Combined in Vitro and in Vivo Analysis of Potential New Biomarkers. Environmental Health Perspectives (Suppl 1): 77–83.
(110) Moriyama, K., Tagami, T., Akamizu, T., Usui, T., Saijo, M., Kanamoto, N., Hataya, Y., Shimatsu, A., Kuzuya, H., and Nakao, K. 2002. Thyroid hormone action is disrupted by bisphenol a as an antagonist. Journal of Clinical Endocrinology and Metabolism 87: 5185–5190.
(111) Olivia, S., Anderson, S., Nahar, S., Faulk, C., Jones, T.R., Liao, C., Kannan, K., Weinhouse, C., Rozek, L.S., and Dolinoy, D.C. 2012. Epigenetic Responses Following Maternal Dietary Exposure to Physiologically Relevant Levels of Bisphenol A. Environmental and Molecular Mutagenesis 53(5): 334–342.
(112) Dolinoy, D.C., Huang, D., and Jirtle, R.L. 2007. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. PNAS 104(32) 13056-13061.
(113) Kim, J.H., Sartor, M. A., Rozek, L.S., Faulk, C., Anderson, O.S., Jones, T.R., Nahar, M.S., and Dolinoy, D.C. 2014. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome, BMC Genomics 15:30.
(114) Cao, J., Rebuli, M.E., Rogers, J., Todd, K.L., Leyrer, S.M., Ferguson, S.A., and Patisaul, H.B. 2013. Prenatal Bisphenol A Exposure Alters Sex-Specific Estrogen Receptor Expression in the Neonatal Rat Hypothalamus and Amygdala. Toxicological Sciences 133(1), 157–173.
(115) Taylor, J.A., Richter, C.A., Suzuki, A., Watanabe, H., Iguchi, T., Coser, K.R., Shioda, T., and vom Saal F.S. 2012. Dose-Related Estrogen Effects on Gene Expression in Fetal Mouse Prostate Mesenchymal Cells. PLoS ONE 7(10): e48311. doi:10.1371/journal.pone.0048311.
(116) Annas, G.J., and Elias, S. 1999. Thalidomide and the Titanic: Reconstructing the Technology Tragedies of the Twentieth Century. American Journal of Public Health 89(1).
(117) Hunt, P.A., VandeVoort, C., Woodruff, T., and Gerona, R. 2014. Invalid controls undermine conclusions of FDA studies. Toxicological Sciences June 3rd, 2014 Advanced Access. DOI:10.1093/toxsci/kfu100
Comments are closed.